On Thursday, the U.S. Food and Drug Administration (FDA) approved a groundbreaking new pain medication, marking the introduction of the first new category of pain relievers in over two decades. The medication, known as suzetrigine, is a prescription pill with a dosage of 50 milligrams, which patients will take every 12 hours following an initial, higher dose. It will be marketed under the brand name Journavx.
Significance of the Approval
Dr. Jacqueline Corrigan-Curay, the FDA’s acting director for the Center for Drug Evaluation and Research, expressed optimism regarding this new non-opioid analgesic. She stated in a news announcement, “A new non-opioid analgesic therapeutic class for acute pain offers an opportunity to mitigate certain risks associated with using an opioid for pain and provides patients with another treatment option.” Corrigan-Curay emphasized that the approval reinforces the FDA's commitment to providing safe alternatives for managing pain.
Pain Medications in the U.S.
According to federal surveys, pain medications, also known as analgesics, rank as the most frequently prescribed drugs in hospitals. A study conducted by Vertex Pharmaceuticals, the developer of suzetrigine, indicates that approximately 80 million Americans receive prescriptions annually for managing acute moderate to severe pain, with nearly half of these prescriptions being for opioids. The widespread use of opioids raises concerns regarding addiction and dependency.
Historical Context
Suzetrigine is particularly noteworthy as it represents the first FDA-approved painkiller since Celebrex, a Cox-2 inhibitor, was introduced in 1998. The mechanism behind suzetrigine is distinct from that of conventional opioid medications. Dr. Sergio Bergese, an anesthesiologist at Stony Brook University, explains that pain is perceived through a pathway involving nerve cells transmitting signals from damaged tissues to the brain. Opioids suppress this perception, whereas suzetrigine prevents pain-signaling nerves from activating.
Unique Mechanism of Action
Dr. Bergese explains, “This drug interrupts that pathway, so even though a tissue injury exists, the brain isn't aware of it.” Importantly, unlike opioids that often induce feelings of euphoria, suzetrigine does not have addictive properties, making it unlikely to lead to dependency among users.
Research Origins
The development of suzetrigine stemmed from research into a family in Pakistan known for their ability to walk barefoot over hot objects without experiencing pain. This phenomenon was traced back to a genetic mutation that inhibited the firing of pain signals in their skin. According to Stuart Arbuckle, chief operating officer of Vertex, while these individuals could feel heat from the coals, the pain-conducting nerves were unaffected. This discovery set off a 25-year journey to develop a medication that exploits this unique pain-conduction mechanism.
How Suzetrigine Functions
Dr. Stephen Waxman, director of the Center for Neuroscience and Regeneration Research at Yale School of Medicine, elaborated that neurons communicate using nerve impulses which are generated by sodium channels, functioning as molecular batteries. Suzetrigine’s innovative approach involves shutting down a specific sodium channel that only transmits pain signals.
Implications for Future Pain Medications
Despite many challenges encountered in developing a targeted pain medication, the approval of suzetrigine is seen as a pivotal advance. Waxman noted, “It is an important step forward, because it provides proof of concept that a [sodium-channel blocker] can reduce pain in humans,” suggesting that this could pave the way for subsequent generations of more effective medications.
Dosing Information
The administration of suzetrigine involves an initial dose of 100 milligrams, followed by 50 milligrams every 12 hours. While promising, healthcare professionals caution that this medication might not be suitable for every individual or type of pain. In clinical trials involving roughly 600 subjects, suzetrigine showed a more effective reduction in pain after abdominal and foot surgeries when compared to a placebo.
Clinical Trial Results
In trials, participants reported a reduction in pain levels, averaging seven out of ten, with suzetrigine alleviating pain by approximately 3.5 points. While the efficacy was comparable to that of Vicodin, a combination of acetaminophen and the opioid hydrocodone, the study design did not directly compare the two medications, leaving some uncertainty in determining which was more effective.
Chronic Pain Considerations
In a separate study focusing on sciatica-related back pain, suzetrigine achieved an average pain reduction of about two points, similar to placebo outcomes, suggesting that it may not be as effective for chronic pain conditions. Vertex Pharmaceuticals counters this claim by asserting that the drug has shown efficacy in various types of long-term pain and continues to explore its application for diabetic neuropathy.
Expert Opinions
Healthcare professionals expressed enthusiasm regarding the addition of a new option for pain management. Dr. Kimberley Mauer, an anesthesiologist from Oregon Health and Science University, emphasized that having more treatment options enhances the ability to cater to individual patient needs. However, the cost of suzetrigine could impact patient access; Vertex has set a wholesale price of $15.50 per 50 mg pill, although patient assistance programs will be available. Mauer noted that insurance coverage remains uncertain, which could further affect patient access to the new medication.
In summary, the FDA’s approval of suzetrigine presents a significant advancement in pain management options that could potentially revolutionize treatment methodologies while addressing concerns surrounding opioid use and its associated risks.
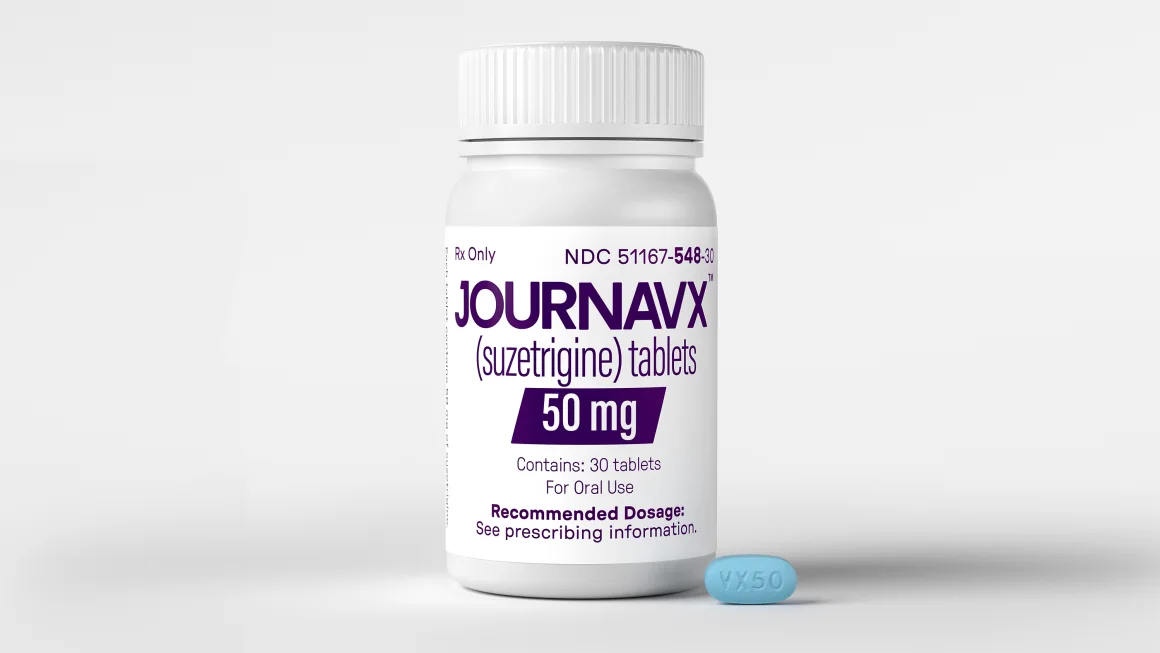 Image Credit: Suzetrigine is a prescription pill taken every 12 hours after a larger starter dose. Vertex Pharmaceuticals
Image Credit: Suzetrigine is a prescription pill taken every 12 hours after a larger starter dose. Vertex Pharmaceuticals